
RepliCel Life Sciences Inc (TSXV:RP)
RepliCel is a regenerative medicine company developing autologous cell therapies that address diseases caused by a deficit of healthy cells required for normal healing and function.
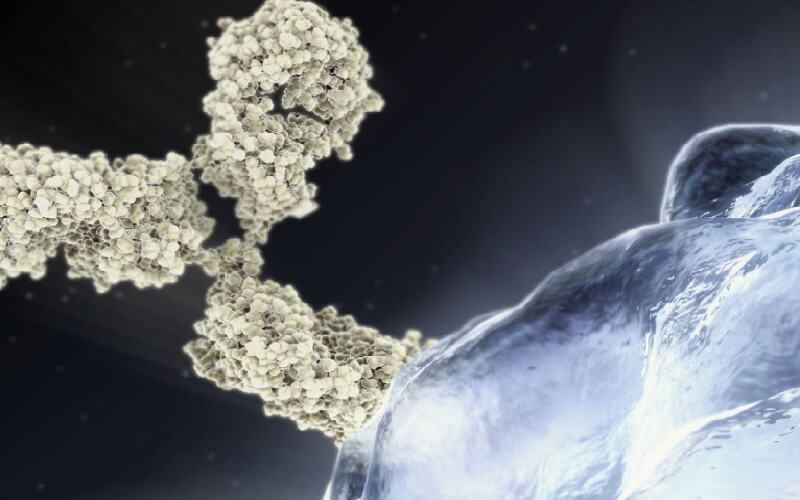
BeiGene, Ltd. (NASDAQ: BGNE) and Boehringer Ingelheim Biopharmaceuticals (China) Ltd. today announced that the two companies have entered into a commercial supply agreement for tislelizumab, BeiGene’s investigational anti-PD-1 antibody. Tislelizumab will be manufactured in Boehringer Ingelheim’s world-class biopharmaceutical manufacturing facility in Shanghai as part of a Marketing Authorization Holder (MAH) trial project pioneered by BeiGene and Boehringer Ingelheim.
“As we advance tislelizumab towards potential regulatory approval and commercialization in China, we are pleased to have secured our commercial product supply from Boehringer Ingelheim BioXcellence, one of the world’s leading biopharmaceutical contract manufacturers. Over the years, working with the Boehringer Ingelheim team for our clinical supply, we have built a strong partnership based on a shared commitment to providing high-quality medicine to patients,” said John V. Oyler, Founder, Chief Executive Officer, and Chairman of BeiGene. “In addition to working with Boehringer Ingelheim, we are also building our manufacturing facility for biologics commercial supply in Guangzhou, China, in a joint venture with the Guangzhou Development District, that we expect will further expand our capacity to meet anticipated demand for tislelizumab and other biologics in our pipeline.”
Under the terms of the supply agreement, Boehringer Ingelheim will manufacture tislelizumab in China under an exclusive multi-year arrangement, with contract extension possible. In addition, BeiGene also obtained certain preferred rights for future capacity expansion by Boehringer Ingelheim in China.
“The agreement marks the first biopharmaceutical MAH trial project entering into commercial supply in China,” said Dr. Jiali Luo, General Manager of Boehringer Ingelheim Biopharmaceuticals (China) Ltd. “Boehringer Ingelheim is focused on manufacturing innovative therapeutics to treat unmet medical needs of patients around the world. We are confident that our quality competence and contract manufacturing capabilities are best-in-class, and excited to plan for the commercial supply of tislelizumab, which has the potential to help patients with a broad array of cancers as both a monotherapy and in combination with other therapies.”
About Tislelizumab (BGB-A317)
Tislelizumab is an investigational humanized monoclonal antibody that belongs to a class of immuno-oncology agents known as immune checkpoint inhibitors. It is designed to bind to PD-1, a cell surface receptor that plays an important role in downregulating the immune system by preventing the activation of T-cells. Tislelizumab has demonstrated high affinity and specificity for PD-1. It is differentiated from the currently approved PD-1 antibodies in an engineered Fc region, which is believed to minimize potentially negative interactions with other immune cells. Tislelizumab is being developed as a monotherapy and in combination with other therapies for the treatment of a broad array of both solid tumor and hematologic cancers. BeiGene and Celgene Corporation have a global strategic collaboration for tislelizumab for solid tumors outside of Asia (except Japan).
Source: thestreet.com