
RepliCel Life Sciences Inc (TSXV:RP)
RepliCel is a regenerative medicine company developing autologous cell therapies that address diseases caused by a deficit of healthy cells required for normal healing and function.
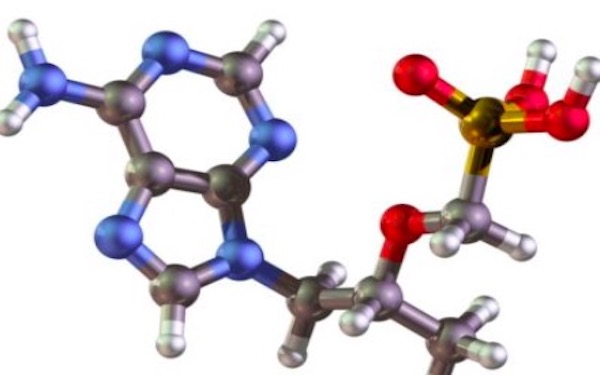
Hutchison China MediTech (Chi-Med) has started a China Phase I trial of its novel spleen tyrosine kinase (Syk) inhibitor as a first line treatment in elderly patients with acute myeloid leukemia. The molecule, HMPL-523, will be administered in combination with an approved nucleoside metabolic inhibitor. In September, Chi-Med’s lead drug, Elunate® (fruquintinib), was approved in China as a third-line treatment for metastatic colorectal cancer, the first China-developed drug approved for a cancer indication.
Source: China Biotoday