
RepliCel Life Sciences Inc (TSXV:RP)
RepliCel is a regenerative medicine company developing autologous cell therapies that address diseases caused by a deficit of healthy cells required for normal healing and function.
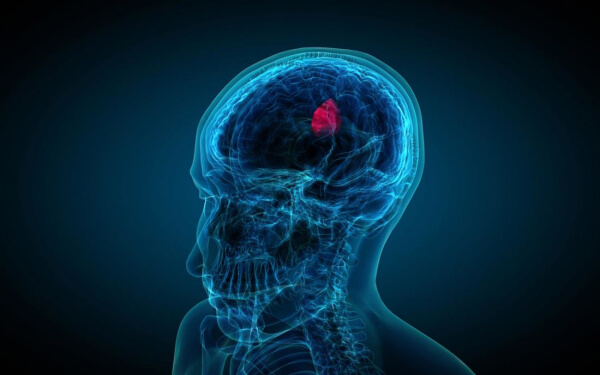
AIVITA Biomedical, Inc., a biotech company specializing in innovative stem cell applications, announced today updated clinical data from its ongoing glioblastoma Phase 2 clinical trial, investigating AIVITA’s platform immunotherapy targeting tumor-initiating cells. Blood plasma biomarker analyses have identified a robust immune response and a decrease of tumor biomarkers in 65% of treated patients, in a sample that represents 29% of the total Phase 2 clinical trial size. Blood was collected from subjects at multiple time points, one week after each dose administration, and assayed for 450 different immune and tumor biomarkers. Treatment elicited a robust immune response, with biomarkers suggesting progressive activation of dendritic cell cross-presentation and progressive activation of a type II hypersensitivity antibody-mediated cytotoxic response. Most notably, 65% of the treated glioblastoma patients also showed a robust decrease of 27 different biomarkers associated with tumor development, including markers of tumor-associated angiogenesis, tumor-associated growth factors, and tumors themselves. “An objective tumor biomarker response, combined with a robust and appropriate immune response, suggests a decrease of tumor burden in 65% of our treated patients,” said Dr. Hans S. Keirstead, AIVITA’s Chief Executive Officer. “Should this translate to survival, it would represent a major step forward in our fight against glioblastoma.” AIVITA is currently conducting three distinct clinical studies investigating its platform immunotherapy, in patients with ovarian cancer, glioblastoma and melanoma. AIVITA uses 100% of proceeds from the sale of its ROOT of SKIN™ skincare line to support the treatment of women with ovarian cancer.