
RepliCel Life Sciences Inc (TSXV:RP)
RepliCel is a regenerative medicine company developing autologous cell therapies that address diseases caused by a deficit of healthy cells required for normal healing and function.
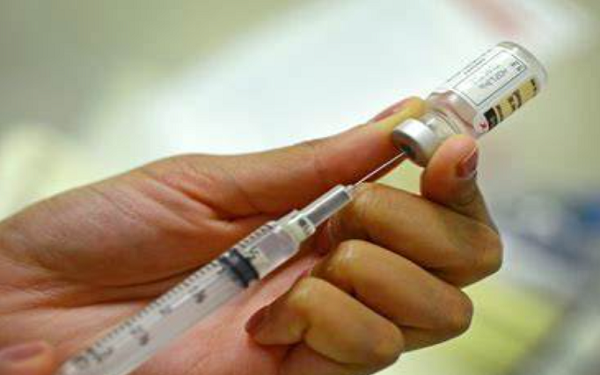
The European Medicines Agency has recommended conditional approval for the COVID-19 vaccine developed by AstraZeneca (AZN -1.2%) in partnership with the University of Oxford in people from 18 years of age.
The experts at EMA noted “the vaccine can be used in older adults,” citing the expected immune protection seen in the age group and based on experience with other vaccines.
However, they have added, “there are not yet enough results in older participants (over 55 years old) to provide a figure for how well the vaccine will work in this group.”
A formal decision from European Commission for the approval is likely in a few hours, potentially making AstraZeneca jab the third COVID-19 shot to receive the approval in the 27-member bloc.
Earlier it was reported that Germany’s vaccine committee had recommended the adenovirus-based vaccine only for people between 18-64 years of age.
eHealth (NASDAQ:EHTH) announces that an affiliate of H.I.G. Capital has entered into a binding agreement to make a $225M investment in the company by purchasing convertible preferred stock.
With the help of the investment, eHealth hopes to accelerate the execution of its strategic initiatives, including driving scale through online enrollment growth and broadening its strategic partner channel among other things.
Upon completion of the investment, which both companies expect to be in Q1 2021, the convertible preferred stock issued to H.I.G. will represent about 8% of eHealth’s common stock.
Moelis & Company LLC is serving as sole placement agent and financial advisor to eHealth and Wilson Sonsini Goodrich & Rosati, Professional Corporation as legal advisor. Ropes & Gray LLP is serving as legal advisor to H.I.G.
Citing the potential of NM-002, an experimental therapy for short bowel syndrome (“SBS”), Oppenheimer starts the coverage on 9 Meters Biopharma (NASDAQ:NMTR) with an Outperform rating and a price target of $6 per share, ~4.4x of today’s close, following a gain of 28.0%.
The analyst Kevin DeGeeter and the team expect the company to launch NM-002 in the US and the EU in 2024, leading to an estimated $727M of sales by 2029 given the convenient dosing regimen and the potential to reduce the frequency and severity of diarrhea.
The company initiated the dosing of NM-002 in a Phase 1b/2a study in adults suffering from SBS in July 2020, expecting the top-line data in Q4 2020.
Despite a twofold rise in share price over the past six-month period, the analysts note that investors have failed to appreciate the opportunity for NM-002 in patients not dependent on parenteral support, a market yet to be addressed by Takeda’s Gattex.
Gattex of Takeda Pharmaceutical (NYSE:TAK) is indicated for adult patients with SBS who are dependent on parenteral support.