
RepliCel Life Sciences Inc (TSXV:RP)
RepliCel is a regenerative medicine company developing autologous cell therapies that address diseases caused by a deficit of healthy cells required for normal healing and function.
DURECT announces Gilead Sciences will terminate the License Agreement in July 2019, effective December 22, for the development and commercialization of a long-acting injectable HIV investigational product utilizing DURECT’s SABER technology.
DURECT anticipates recognizing remaining deferred revenue of ~$23.1M Q2 of 2020, associated with the receipt of the upfront license and development milestone payment.
(NASDAQ:DRRX) is down ~1% in after hours.
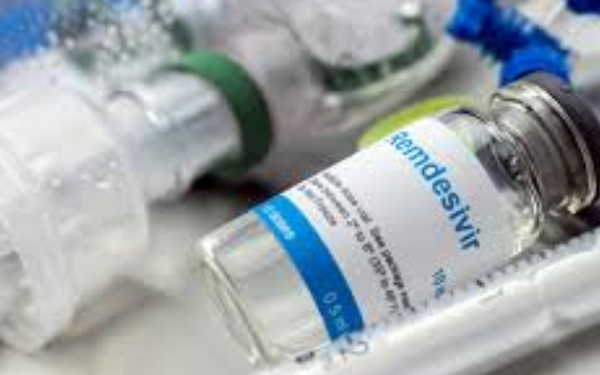
According to the U.S. Department of Health and Human website, the federal government will send more vials of Gilead Sciences’ (GILD -1.2%) remdesivir to states experiencing high rates of COVID-19 infections. Shipments will start on Monday, June 29.
California will receive 464 cases, each containing 40 vials. Texas will receive 448 cases, Florida 360 and Arizona 356.
The company donated its entire inventory (~1.5M doses) of the antiviral when it received the emergency use nod from the FDA on May 1.
The FDA approves Chiasma’s (CHMA -1.5%) Mycapssa (octreotide) for the long-term maintenance treatment of adult patients with acromegaly, a disorder in which the pituitary gland produces excessive amounts of growth hormone which causes bones in the hands, feet and face to increase in size.
Management will host a conference call today at 2:00 pm ET to discuss the nod.
Shares, currently halted, have yet to resume trading.
Update: Commercial launch expected in Q4.
Zogenix (ZGNX -3.7%) has experienced substantial volatility today after the FDA announced approval of Fintepla (fenfluramine) for a rare type of epilepsy called Dravet syndrome after the close yesterday, normally a bullish event despite broad market behavior.
Shares jumped early, up as much as 15% reaching an intraday high of $32.42 before reversing, dropping as low as $24.60 (-13%). Turnover is ~8.2M shares, 10x normal.
The drug will go head-to-head with GW Pharmaceuticals’ (GWPH -4.1%) Epidiolex (cannabidiol), approved in the U.S. two years ago for Dravet (and Lennox-Gastaut syndrome).
On the efficacy front, treatment with Fintepla resulted in a drop in median convulsive seizure frequency as high as 74.9% (Study 1) (two-week titration period followed by 12-week treatment period) compared to a drop of 39% for Epidiolex during a 28-day treatment period.
Investors appear to be reacting to the boxed warning in Fintepla’s labeling stating that it is associated with valvular heart disease (VHD) and pulmonary arterial hypertension (PAH). Treated patients must have echocardiograms every six months during treatment and once, within 3-6 months, after treatment is discontinued. In its most recent corporate presentation, Zogenix stated that there were no cases of VHD or PAH observed in over 700 echos performed in its trials.
In a late-night court filing, the Trump administration urged the Supreme Court to overturn the Affordable Care Act in a case that’s set to be heard around the time of the November election.
The brief argues that because the law’s requirement to have health insurance was upheld in court as a tax in 2012 – and Congress has since repealed the financial penalty for violating that requirement in 2017 – it is no longer a tax and therefore no longer constitutional.
“The entire ACA thus must fall with the individual mandate, though the scope of relief entered in this case should be limited to provisions shown to injure the plaintiffs,” Solicitor General Noel Francisco wrote in the filing.
Related tickers: UnitedHealth (NYSE:UNH), Anthem (NYSE:ANTM), Cigna (NYSE:CI), Humana (NYSE:HUM), WellCare Health Plans (NYSE:WCG), Centene (NYSE:CNC), Molina Healthcare (NYSE:MOH), Triple-S Management (NYSE:GTS), Civitas Solutions (NYSE:CIVI), CVS Health (NYSE:CVS), Cardinal Health (NYSE:CAH), McKesson (NYSE:MCK), AmerisourceBergen (NYSE:ABC), Universal Health Services (NYSE:UHS), LifePoint Health (NASDAQ:LPNT), Community Health Systems (NYSE:CYH), HCA Healthcare (NYSE:HCA), HealthEquity (NASDAQ:HQY), Quorum Health (NYSE:QHC) and Tenet Healthcare (NYSE:THC).