
RepliCel Life Sciences Inc (TSXV:RP)
RepliCel is a regenerative medicine company developing autologous cell therapies that address diseases caused by a deficit of healthy cells required for normal healing and function.
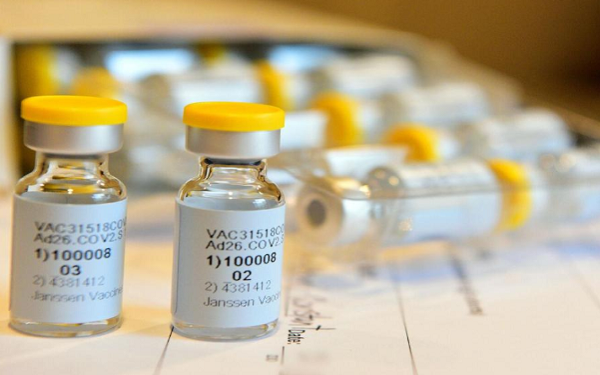
The non-peer reviewed results say 98% of participants with available data had neutralizing antibodies 29 days after a single shot vaccination from candidate Ad26.COV2.S.
Among the caveats is that results were available from only 15 participants above the age of 65. For those over 65, the rate of adverse reactions was 36% vs. 64% for all others, suggesting a lessened immune response.
NAI500 seeks to provide the most pressing investment knowledge to its readers. On October 7 and 8, NAI500 is hosting a FREE VIRTUAL EVENT focusing on the rapidly growing mining sector. Learn about Gold and Precious Metals Exploration, Battery Metals and much more by registering here.
Based on these results, J&J (NYSE:JNJ) on Wednesday began a Phase 3 trial on 60K participants, with results expected by early next year.
As expected, the European Commission approves Gilead Sciences’ (GILD -0.5%) and collaboration partner Galapagos NV’s (GLPG +3.2%) Jyseleca (filgotinib) for adults with moderate-to-severe rheumatoid arthritis who have failed to respond to or are intolerant of one or more disease-modifying anti-rheumatic drugs.
Two months ago, the advisory group CHMP adopted a positive opinion backing the nod.
The JAK1 inhibitor was just approved in Japan for RA, but the companies are not quite there in the U.S. The FDA wants to see data from two ongoing trials, expected in H1 2021.
Quant rating on GLPG is Very Bearish.
Generex Biotechnology’s (OTCQB:GNBT +18.0%) newly formed subsidiary NuGenHealth has signed a contract with Paradise Valley Family Medicine for providing connected care solutions for patient engagement, Remote Patient Monitoring and Chronic Care Management services.
NuGenHealth is a JV between Generex subsidiary NuGenerex Health and Worldwide Digitech.
The NuGenHealth system offers direct savings to physicians and healthcare organizations both in time and money.
“And once NuGenHealth is established in the practices, we plan to launch our long-awaited ophthalmology and podiatry businesses to serve the large numbers of patients with diabetes and vascular conditions that are prevalent in the chronic care population,” president & CEO Joe Moscato commented.
Endo International (NASDAQ:ENDP) subsidiary, Par Sterile Products enters into a non-exclusive agreement with Novavax (NASDAQ:NVAX) to provide fill-finish manufacturing services at its plant in Rochester, Michigan for NVX-CoV2373, Novavax’ COVID-19 vaccine candidate.
The Rochester facility has begun production of NVX-CoV2373 final drug product, with initial batches to be used in Novavax’ pivotal Phase 3 clinical trial in the US.
NVAX +5.7% PM; ENDP +5.3% PM
Japan’s Ministry of Health, Labor and Welfare has approved Gilead’s (NASDAQ:GILD) Jyseleca (filgotinib 200 mg and 100 mg tablets), a once-daily oral JAK1 inhibitor, for the treatment of rheumatoid arthritis (RA) patients who did not respond adequately to conventional therapies.
Gilead will hold the marketing authorization of Jyseleca in Japan and supply the product, while Eisai (OTCPK:ESALF) will manage distribution.
Gilead is developing Jyseleca in collaboration with Galapagos (NASDAQ:GLPG).
Last month, Galapagos and Gilead announced FDA CRL for filgotinib for RA as the agency requested for more data and review period extended into 2021.